Beyond the Neuron: Myelination’s Role in Disorder and Disease
Author: Matthew Calderone || Scientific Reviewer: Nakiyah Shoemake || Lay Reviewer: Yanna O'Boyle || General Editor: Margaret Silva
Artist: Gauri Kumar || Graduate Scientific Reviewer: Rachel Podgorski
Publication Date: December 20th, 2022
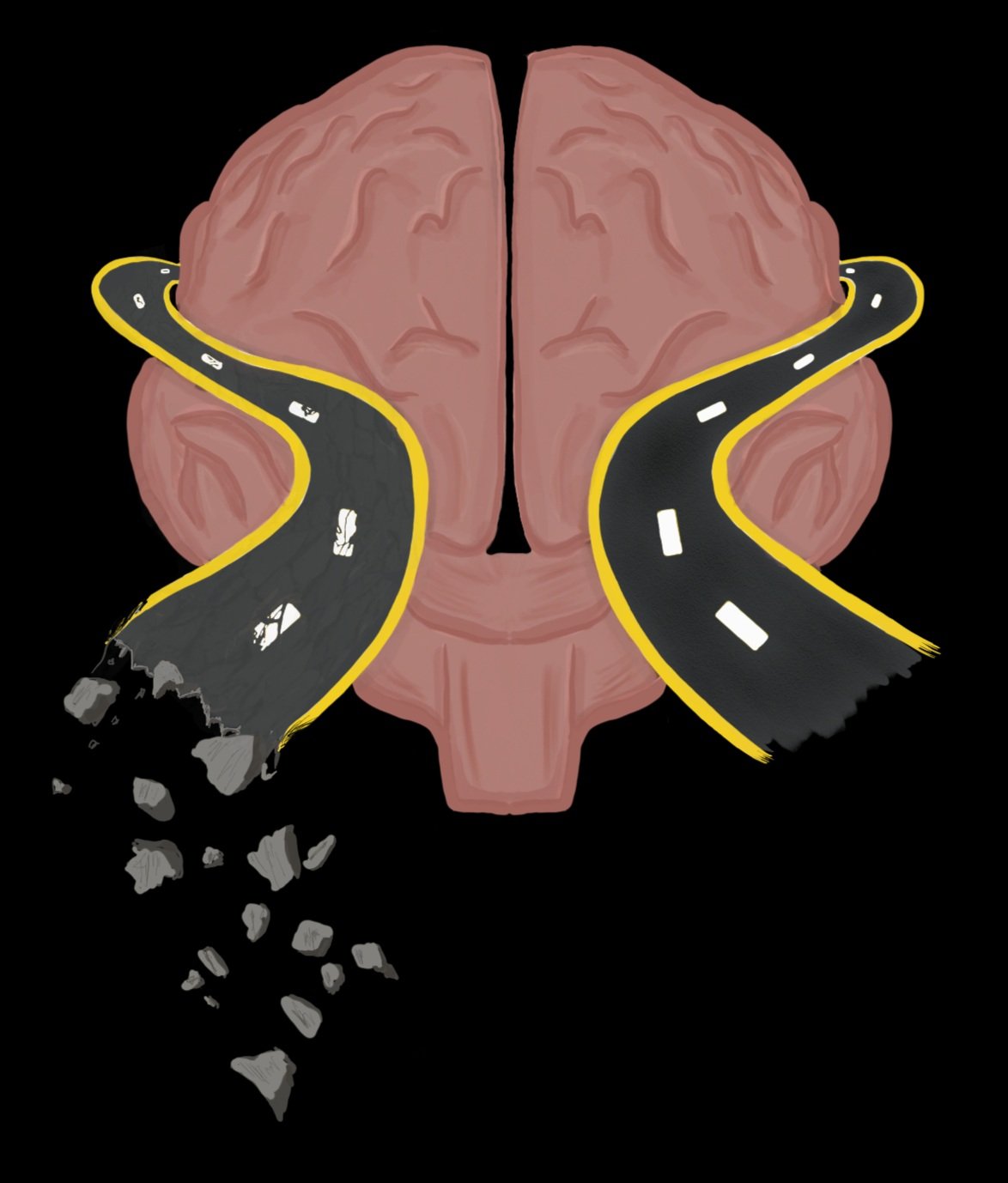
Although neurons receive a majority of the attention in widespread neuroscience literature, there are many cell types in the brain that have crucial roles in regulating brain function. One of these cell types, glial cells, account for 33% of all brain tumors and 50% of all cells in the brain [1, 2]. Glial cells, also referred to as glia, play a role in pathogenesis of diseases and disorders of the central nervous system, which consists of both the brain and spinal cord. Glia come in three subsets: microglia, astrocytes, and oligodendrocytes. Microglia, named as such for their small size, are major players in the brain’s immune system. Astrocytes (which means star [astro] cell [cyte]) are responsible for transportation of nutrients [3]. The third glial cell type, Oligodendrocytes, are unique because they have complex, specific interactions with neurons. To illustrate these interactions, imagine you are driving a car down a straight dirt path in the countryside, with occasional potholes and pebbles that are getting kicked up while you are driving. Now compare this to driving on a newly constructed, freshly paved stretch of highway on the Pennsylvania turnpike. What can you assume about safe speeds of the cars in these two situations? What about the duration of the trip? This example highlights two key features applicable within the brain. The average speed of your car is proportional to the condition of the road, meaning that if the road is smooth and paved, your car can drive faster, and the duration of your trip is inversely proportional to these road conditions, meaning that if the road is rough and unpaved, your trip is longer. In this sense, neurons are like roads; they can be “paved” or myelinated, a term used to describe when oligodendrocytes coat or wrap around a portion of the neuron to create a covering called the myelin sheath [4].
Essentially, myelination converts the communication space between neurons into a super-highway. Before it can send its own signal, a neuron must receive information through its dendrites. Dendrites are branchlike extensions from the neuron’s cell body and relay the signal all the way to its end, the axon. Once the axon receives this signal, it is able to produce an electrical current that causes a cascade of cellular processes within the neuron. This cascade results in the release of signaling molecules from the axon into the synaptic cleft, the space between one neuron’s axon and the dendrite of the receiving neuron. Myelination increases the efficiency of a neuron’s axon, making the process of transmitting signals easier and faster. This is why we are able to respond quickly to our environment, and why you’re able to focus your eyes on the words you’re reading now. Myelination plays a key role in ensuring proper functioning of the brain’s circuits and pathways, and when this system doesn’t function properly it can lead to disastrous effects.
As myelination occurs many times throughout many regions of the central nervous system; this means there are many opportunities for errors to occur. Two examples of this are demyelination and dysmyelination. Demyelination is a process by which the myelin sheath degrades, while the axon is left mostly intact [5]. Dysmyelination is a process in which neurons are not properly myelinated. The distinction between demyelination and dysmyelination is that demyelination occurs in neurons that were originally myelinated, whereas in dysmyelination, neurons never had proper myelination to begin with.
Multiple sclerosis (MS) is a disorder that causes the immune system to attack and degrade myelin of the central nervous system, and is a prime example of demyelination resulting from a disorder [6]. MS mainly affects young adults, primarily those between the ages of 20 and 40 years of age, and presents in two forms; relapsing remitting MS (RRMS), and less commonly, secondary progressive multiple sclerosis [7]. Typically, the first stage of MS is RRMS, where a patient may have episodes of MS symptoms consisting of: tingling of the extremities, visual impairments, fatigue, spascity, urinary dysfunction, and learning and memory impairments [7,8].These symptoms develop over hours to days and eventually hit a plateau where they will persist for several weeks then eventually desist [7]. Recovery from these episodes comes with a rebound of symptoms, but magnetic resonance imaging (MRI), a non-invasive technique used to build 3D images of organs, reveals asymptomatic lesions not only in the brain, but also within the spinal cord [9, 10].These lesions, regions of damaged cells, contribute to continued neurodegeneration and worsening of symptoms to the point of secondary progressive MS, a disease state where the progression of the disease is no longer halted by remitted periods of symptoms [9].
Another disorder that causes demyelination is acute disseminated encephalomyelitis (ADEM) which is characterized by a rapid, short-lasting inflammation that destroys myelin. While the reason for this attack is not completely known, current theories link the inflammation to environmental stress in genetically vulnerable people [11]. ADEM can be mistaken for an MS episode but comes with its own differentiating properties. ADEM is most common in children under the age of 10, and typically presents as a single widespread attack on the myelin sheath. In contrast, MS is characterized by persistent attacks on the sheath spanning over the course of a few weeks or months and occurs as a result of viral or bacterial infections [11, 12]. These two disorders are classic examples and give important insight into disorders associated with improper myelination.
Interestingly, there are emerging theories about myelin’s potential role in previously unlinked disorders. Some of these theories look at the role of myelin in attention-deficit/hyperactivity disorder (ADHD), autism spectrum disorder (ASD), obsessive-compulsive disorder (OCD), and post-traumatic stress disorder (PTSD). ADHD is a neurodevelopmental disorder, meaning it arises during the early stages of brain development, and is characterized by a dysregulation of the reward system within the brain [13]. Behaviorally, ADHD is seen as inattention, hyperactivity, and a lack of executive functioning skills for the patient’s age [14]. Genome-wide association meta-analyses have recently been employed to scan the genetic library of individuals with ADHD in an attempt to find its genetic components [15, 16]. These analyses have found mutations in a gene that encodes for the beta-galactoside-alpha-2,3-sialyltransferase-III membrane protein (ST3GAL3). Silencing of this protein in mice, by knockout of two exons (the coding region of DNA) within the gene’s coding sequence, has been linked to a cognitively impaired behavioral phenotype caused by dysmyelination [17].
ASD is another neurodevelopmental disorder characterized by difficulties in communication, restricted interests, repetitive behaviors, and other symptoms that impair a person’s social integration [18]. While there is a genetic component to ASD, the precise localization of the causes of the disorder are not well characterized. Phan et al. performed an analysis of differentially expressed genes (DEGs) and linked ASD pathology to genes dysregulating oligodendrocytes, or in our terms, dysmyelination [19]. This dysregulation of myelination can be seen in human post-mortem brain tissue as well as in ASD mouse models, and opens the door for further research.
In contrast, OCD is a chronic, long-lasting disorder which consists of uncontrollable obsessive thoughts and compulsive behaviors [20]. An MRI study investigating the brain composition of OCD patients found that while there was no difference in total brain volume or gray matter volumes (neurons), as compared to control brains, the brains of OCD patients were found to have higher total white matter volumes (glia) [21]. This increase of myelin could be quickening the connection between compulsive behavior and obsessive thought modulating portions of the brain.
Similarly, Chao et al., performed MRI analyses in veterans suffering from PTSD, in comparison to matched trauma-controlled veterans not suffering from PTSD. These analyses found significantly more myelination of the hippocampus, the memory center of the brain [22]. OCD and PTSD highlight an interesting break from the previously mentioned component of demyelination in MS, and ADEM, as well as the dysmyelination seen in ADHD and ASD. That is the problem of over-myelination, an issue which can be thought of as reinforcing a negative pathway through the brain, meaning to create a quicker reaction to negative stimuli; this results in stress, obsessions, and compulsions.
Overall, myelin’s role within the brain is to streamline neuronal signals. Issues in this process by demyelination or dysmyelination can lead to problematic transduction of these signals. Two disorders that characterize demyelination are MS and ADEM, the distinction being repeated attacks in MS, and a single attack in ADEM. ADHD and ASD are neurodevelopmental disorders with genetic components linked to dysmyelination by way of insufficient myelination. PTSD, and OCD are less genetically associated disorders that relate to dysmyelination through increased levels of myelination; mechanistically this could be through quicker response to negative stimuli. Myelination is a process that is crucial for the proper functioning of neuronal pathways throughout the brain, and alterations to this delicate process can lead to devastating effects.
References:
Gliomas. Johns Hopkins Medicine. (2022, February 11). Retrieved September 16, 2022, from https://www.hopkinsmedicine.org/health/conditions-and-diseases/gliomas
von Bartheld, C. S., Bahney, J., & Suzana Herculano-Houzel. (2016, May 17). The search for true numbers of neurons and glial cells in the human brain: A review of 150 years of cell counting. Wiley Online Library. Retrieved September 16, 2022, from https://onlinelibrary.wiley.com/doi/10.1002/cne.24040
Jäkel, S., & Dimou, L. (2017). Glial cells and their function in the Adult Brain: A journey through the history of their ablation. Frontiers in Cellular Neuroscience. https://doi.org/10.3389/fncel.2017.00024
Bradl, M., & Lassmann, H. (2009). Oligodendrocytes: Biology and pathology. Acta Neuropathologica, 119(1), 37–53. https://doi.org/10.1007/s00401-009-0601-5
Love, S. (2006). Demyelinating diseases. Journal of Clinical Pathology, 59(11), 1151–1159. https://doi.org/10.1136/jcp.2005.031195
Lemus, H. N., Warrington, A. E., & Rodriguez, M. (2018). Multiple sclerosis. Neurologic Clinics, 36(1), 1–11. https://doi.org/10.1016/j.ncl.2017.08.002
Dobson, R., & Giovannoni , G. (2018, October). Multiple sclerosis – a review - Dobson - Wiley Online Library. Wiley Online Library . Retrieved September 29, 2022, from https://onlinelibrary.wiley.com/doi/abs/10.1111/ene.13819
Ghasemi, N., Razavi, S., & Nikzad, E. (2017). Multiple sclerosis: Pathogenesis, symptoms, diagnoses and cell-based therapy. Cell journal. Retrieved November 11, 2022, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5241505/
Thompson , A., Banwell, B. L., Barkhof, F., Carroll, W. M., Coetzee, T., Comi, G., Correale, J., Fazekas, F., Filippi , M., Freedman, M. S., Fujihara, K., Galetta, S. L., Hartung , H. P., Kappos, L. , Lublin, F. D., Marrie, R. A., Miller, A. E., Miller, D. H., Montalban , X., … Cohen, J. A. (2017, December). Diagnosis of multiple sclerosis: 2017 revisions of the McDonald Criteria. The Lancet. Neurology. Retrieved November 29, 2022, from https://pubmed.ncbi.nlm.nih.gov/29275977/
U.S. Department of Health and Human Services. (n.d.). Magnetic Resonance Imaging (MRI). National Institute of Biomedical Imaging and Bioengineering. Retrieved November 29, 2022, from https://www.nibib.nih.gov/science-education/science-topics/magnetic-resonance-imaging-mri
Anilkumar, A. C., Tadi, P., & Foris, L. A. (2022, January). Acute Disseminated Encephalomyelitis. National Library of Medicine. Retrieved November 12, 2022, from https://www.ncbi.nlm.nih.gov/books/NBK430934/
U.S. Department of Health and Human Services. (n.d.). Acute disseminated encephalomyelitis. National Institute of Neurological Disorders and Stroke. Retrieved September 28, 2022, from https://www.ninds.nih.gov/health-information/disorders/acute-disseminated-encephalomyelitis
Stark, R., Bauer, E., Merz, C. J., Zimmermann, M., Reuter, M., Plichta, M. M., Kirsch, P., Lesch, K. P., Fallgatter, A. J., Vaitl, D., & Herrmann, M. J. (2011). ADHD related behaviors are associated with brain activation in the reward system. Neuropsychologia, 49(3), 426–434. https://doi.org/10.1016/j.neuropsychologia.2010.12.012
Centers for Disease Control and Prevention. (2022, August 9). What is ADHD? Centers for Disease Control and Prevention. Retrieved November 12, 2022, from https://www.cdc.gov/ncbddd/adhd/facts.html#:~:text=ADHD%20is%20one%20of%20the,)%2C%20or%20be%20overly%20active.
Demontis, D., Walters, R. K., Martin, J., Mattheisen, M., Als, T. D., Agerbo, E., Baldursson, G., Belliveau, R., Bybjerg-Grauholm, J., Bækvad-Hansen, M., Cerrato, F., Chambert, K., Churchhouse, C., Dumont, A., Eriksson, N., Gandal, M., Goldstein, J. I., Grasby, K. L., Grove, J., … Neale, B. M. (2018, November 26). Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nature News. Retrieved November 11, 2022, from https://www.nature.com/articles/s41588-018-0269-7
Lesch, K.-P. (2019, February 20). Editorial: Can dysregulated myelination be linked to ADHD pathogenesis ... Association for Children and Adolesent Mental Health. Retrieved October 7, 2022, from https://acamh.onlinelibrary.wiley.com/doi/10.1111/jcpp.13031
Yoo, S. W., Motari, M. G., Susuki, K., Prendergast, J., Mountney, A., Hurtado, A., & Schnaar, R. L. (2015). Sialylation regulates brain structure and function. The FASEB Journal, 29(7), 3040–3053. https://doi.org/10.1096/fj.15-270983
U.S. Department of Health and Human Services. (n.d.). Autism spectrum disorder. National Institute of Mental Health. Retrieved November 12, 2022, from https://www.nimh.nih.gov/health/topics/autism-spectrum-disorders-asd
Phan, B. D. N., Bohlen, J. F., Davis, B. A., Ye, Z., Chen, H.-Y., Mayfield, B., Sripathy, S. R., Page, S. C., Campbell, M. N., Smith, H. L., Gallop, D., Kim, H., Thaxton, C. L., Simon, J. M., Burke, E. E., Shin, J. H., Kennedy, A. J., Sweatt, J. D., Philpot, B. D., … Maher, B. J. (2020, February). A myelin-related transcriptomic profile is shared by Pitt-Hopkins Syndrome Models and human autism spectrum disorder. Nature neuroscience. Retrieved October 7, 2022, from https://pubmed.ncbi.nlm.nih.gov/32015540/
U.S. Department of Health and Human Services. (n.d.). Obsessive-compulsive disorder: When unwanted thoughts or repetitive behaviors take over. National Institute of Mental Health. Retrieved November 12, 2022, from https://www.nimh.nih.gov/health/publications/obsessive-compulsive-disorder-when-unwanted-thoughts-take-over
Atmaca, M., Onalan, E., Yildirim, H., Yuce, H., Koc, M., & Korkmaz, S. (2010). The Association of Myelin oligodendrocyte glycoprotein gene and white matter volume in obsessive–compulsive disorder. Journal of Affective Disorders, 124(3), 309–313. https://doi.org/10.1016/j.jad.2010.03.027
Chao, L. L., Tosun, D., Woodward, S. H., Kaufer, D., & Neylan, T. C. (2015). Preliminary evidence of increased hippocampal myelin content in veterans with posttraumatic stress disorder. Frontiers in Behavioral Neuroscience, 9. https://doi.org/10.3389/fnbeh.2015.00333