Levodopa and Parkinson's
Author: Ekta Arora || Scientific Reviewer: Anushka Gangupanthulu || Lay Reviewer: Hannah Joseph || General Editor: Jam Stebbins
Artist: Aya Sadibekova || Graduate Scientific Reviewer: Michael Levin
Publication Date: December 18th, 2023
Parkinson’s Disease is a neurological disorder that causes tremors, slow movement, and stiffness. The condition often progresses relative to the time of diagnosis, since the worsening of symptoms is largely due to the natural decrease of dopamine levels as a patient ages [1]. The exact cause of Parkinson’s is unknown due to its heterogeneity which makes it a complex disease. There has been speculation about whether Parkinson’s Disease has genetic factors. Current research shows that there are 548 known genetic loci that are involved in Parkinson’s [2]. Furthermore, research has shown that Parkinson’s may be brought on by exposure to toxins in insecticides such as rotenone and permethrin, and factors such as head trauma [3].
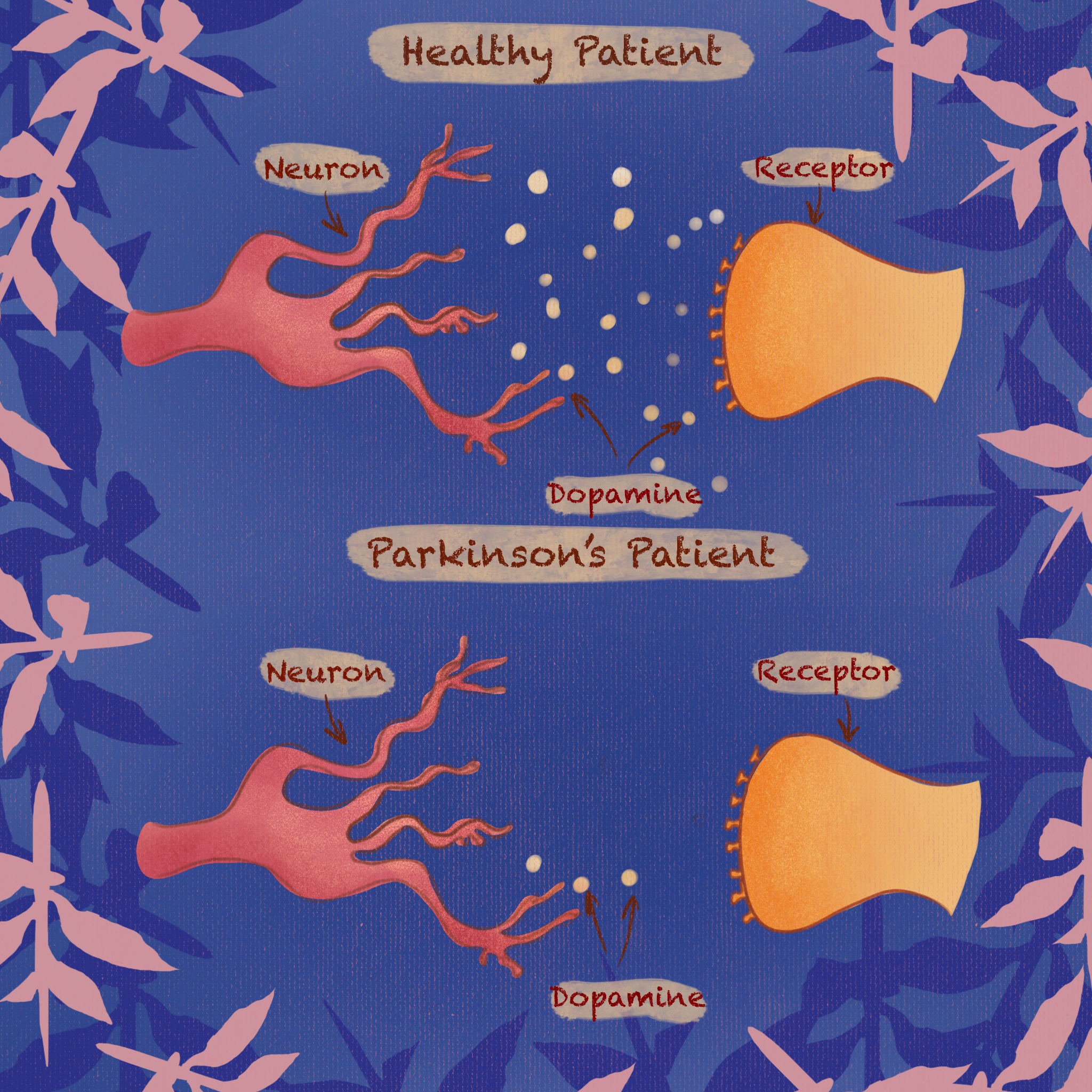
On a molecular level, Parkinson’s Disease is caused by the malfunction of dopamine-secreting neurons as well as neurons that have dopaminergic receptors within the brain. Dopamine is essential for physical movements, as it acts as a trigger in the brain by binding to receptors within the cerebral cortex. These receptors control fine motor function. In Parkinson’s, due to the loss of dopamine, normal binding processes can not be maintained, resulting in the loss of fine motor skills. Imaging of the dopamine reception system in the brain can be conducted through positron emission tomography (PET) scans as well as single photon emission computed tomography (PECT) scans. These scans are then used to visualize the dopamine reception network in the brain and compare structural differences to those of healthy brains [4].
While Parkinson’s Disease is chronic, there are effective treatments to combat and manage symptoms. Levodopa was first clinically tested in 1961 and approved by the FDA in 1970 [5]. Levodopa, also known as L-Dopa, is a widely accepted treatment for Parkinson’s Disease. L-Dopa is a precursor molecule to dopamine that allows the body to stabilize its dopamine levels in order to minimize symptoms [6]. A precursor molecule is a compound that participates and aids in the production of another molecule. L-Dopa supplements the brain with a dopamine precursor compound which stimulates the activation of dopaminergic receptors. Dopaminergic receptors are located on the surface of cells in the brain that are involved with processes that include dopamine, such as motor control, emotion, and executive function. In healthy individuals, dopaminergic receptors are typically stimulated by endogenous, or internally derived, dopamine [7]). However, patients with Parkinson’s do not see as efficient dopamine reception. To combat this medication can be taken orally. L-Dopa then crosses the blood-brain barrier, which is a protective layer of cells that enables specific molecules and ions to pass between the blood and the brain [6].
L-Dopa can be used in combination with other medications in certain individuals that experience acute Parksinon’s symptoms such as the loss of fine motor function. Due to L-Dopa’s quick breakdown within the body, additional medications are used to increase the effective duration of L-dopa. One such medication is Carbidopa, which is used to inhibit the decarboxylation of L-Dopa to dopamine, causing elevated levels of the medication, which is essential for fine motor skills and nervous system functions [6]. Dopamine decarboxylase inhibitors prevent the conversion of L-Dopa to dopamine in the periphery, allowing for more L-Dopa to cross the blood-brain barrier. Decarboxylation refers to the replacement of a carboxyl group with a hydrogen atom; this process leads to the synthesis of dopamine from precursor molecules such as L-dopa.Side effects caused by L-Dopa, such as dyskinesia, the involuntary movement of the face, arms, or trunk. These symptoms worsen with the duration of medication use. Additionally, the distribution of L-Dopa has the tendency to be unevenly distributed due to the gastric process, which individuals often opt to postpone treatment. Treatment with L-Dopa should be limited to the lowest effective dose at the early stages of Parkinson’s disease with the progression being a driving force to increase dosage.
In many cases, if symptoms are mild, patients will often opt for other drugs such as dopamine agonists. Dopamine agonists are a class of Parkinson’s medication that stimulate regions of the brain which typically receive dopamine signals. This tricks the brain into thinking it is receiving the needed amounts of dopamine. While they are not as potent as L-Dopa and Carbidopa, dopamine agonists are an effective option for those with milder forms of Parkinson’s disease as they do not cause strong dyskinesia symptoms [7].
Medications such as L-Dopa, Carbidopa, and the class of dopamine agonists are important for treating Parkinson’s Disease. Upcoming Parkinson’s medication research aims to minimize the side effects of medication such as dyskinesia as well as prevent any drug-related progression of the disease. For example, a long-term issue for oral tablet L-Dopa and Carbidopa users has been the fluctuation in drug levels within the body. This has led researchers to develop other methods of administering medication. One new method of drug administration called Neuroderm, or ND0612, is a long-release topical treatment method. Neuroderm balances medication levels throughout the body by bypassing the need for gastric involvement that arises from oral medication usage, which is often the source of uneven distribution of medication due to the digestive process [8]. Neuroderm provides a continuous release of liquid L-Dopa and Carbidopa subcutaneously, or through the skin, in order to balance dopamine levels [8]. While L-Dopa and Carbidopa are similar in efficacy, research continues to develop more effective ways to administer medication and control the body's response.
While Parkinson’s Disease still remains complex, without any known causes and thus clinically incurable. However, modern research advancements have allowed for effective management of the disease. With the emergence of breakthrough treatments like Neuroderm, the future for Parkinson’s patients looks bright.
References
Fahn, S., Oakes, D., Shoulson, I., Kieburtz, K., Rudolph, A., Lang, A., Olanow, C. W., Tanner, C., & Marek, K. (2004, December 9). Levodopa and the progression of parkinson’s disease. The New England journal of medicine. https://pubmed.ncbi.nlm.nih.gov/15590952/
Hastings, E., & Burdett, T. (n.d.). Trait: Parkinson disease. GWAS catalog. https://www.ebi.ac.uk/gwas/efotraits/MONDO_0005180
Can environmental toxins cause parkinson’s disease? John Hopkins Medicine. (n.d.). https://www.hopkinsmedicine.org/health/conditions-and-diseases/parkinsons-disease/can-environmental-toxins-cause-parkinson-disease
Hauser, R. A., & Grosset, D. G. (2011, March 16). [123i]fp‐cit (DaTscan) Spect Brain Imaging in ... - wiley online library. Wiley Online Library. https://onlinelibrary.wiley.com/doi/10.1111/j.1552-6569.2011.00583.x
Hauser, R. A. (2009, June). Levodopa: Past, Present, and Future. Karger Publishers. https://karger.com/ene/article/62/1/1/124490/Levodopa-Past-Present-and-Future
Abbott, A. (2010, August 25). Levodopa: The story so far. Nature News. https://www.nature.com/articles/466S6a
Choi, J., & Horner, K. A. (2023, June 26). Dopamine agonists - statpearls - NCBI BOOKSHELF. National Library of Medicine. https://www.ncbi.nlm.nih.gov/books/NBK551686/
LeWitt, P. A., Stocchi, F., Arkadir, D., Caraco, Y., Adar, L., Perlstein, I., Case, R., & Giladi, N. (2022, November 10). The pharmacokinetics of continuous subcutaneous levodopa/carbidopa infusion: Findings from the ND0612 Clinical Development Program. Frontiers in neurology. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9686322/